research target:miR-223
Periodicals:Journal of clinical investigation
IF:13.251
Cooperative Unit:Guangzhou Women and Children's Medical Center
Time of publication:January,2019

Summary
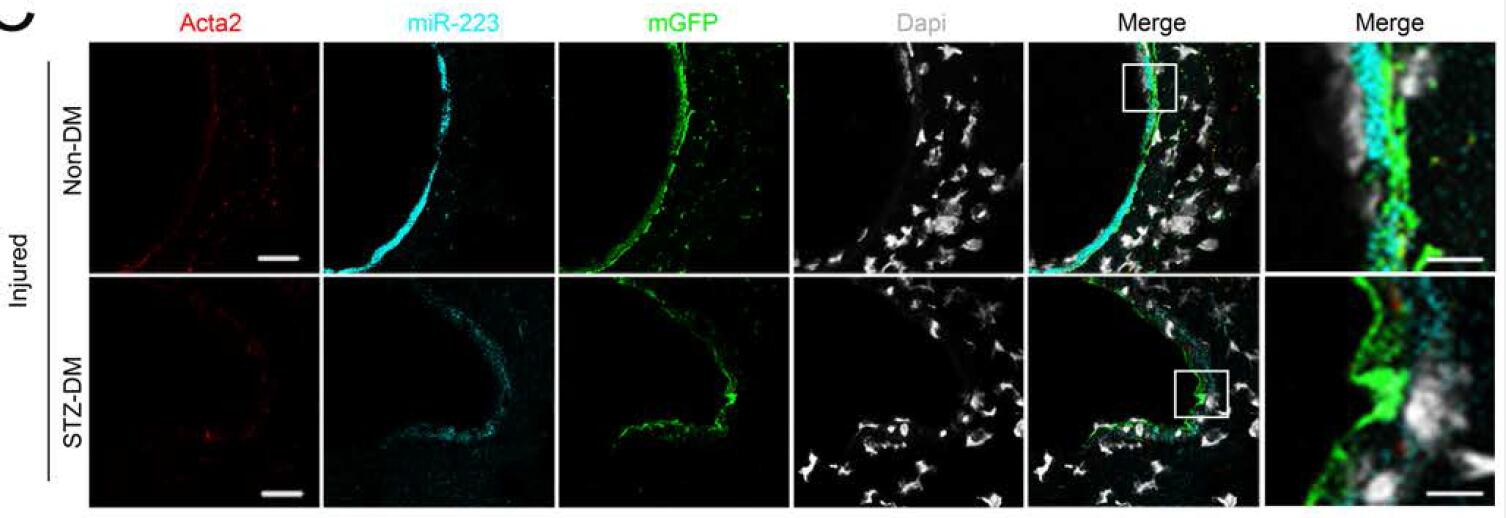
Upon arterial injury, endothelial denudation leads to platelet activation, and delivery of multiple agents (e.g. TXA2, PDGF) promoting VSMC dedifferentiation, and proliferation, in injury repair (intimal hyperplasia). Resolution of vessel injury repair, and prevention of excessive repair (switching VSMC back to a differentiated quiescent state) is a poorly understood process. We now report that internalization of activated platelets by VSMCs promotes resolution of arterial injury by switching on VSMC quiescence. Ex vivo and in vivo studies using lineage tracing reporter mice (PF4-Cre x mTmG) demonstrated uptake of green platelets by red vascular smooth muscle cells upon arterial wire injury. Genome-wide miRNA sequencing of VSMCs co-cultured with activated platelets identified significant increases in platelet-derived miR-223. miR-223 appears to directly target PDGFRβ (in VSMCs) reversing the injury-induced dedifferentiation. Upon arterial injury platelet miR-223 knockout mice exhibit increased intimal hyperplasia, whereas miR-223 mimics reduced intimal hyperplasia. Diabetic mice with reduced expression of miR-223, exhibited enhanced VSMC dedifferentiation, proliferation and increased intimal hyperplasia. Horizontal transfer of platelet-derived miRNAs into VSMCs provide a novel mechanism for regulating VSMC phenotypic switching. Platelets thus play a dual role in vascular injury repair, initiating an immediate repair process, and concurrently, a delayed process to prevent excessive repair..
Key words: Vascular smooth muscle cells, arterial injury, platelets, diabetes mellitus, microRNA.
Partial results of cooperation
BersinbioTM cooperative technology:miRNA probe, FISH
Original link:https://www.jci.org/articles/view/124508

